Therapeutic Mouthrinses:
Today, patients and their dental professionals are faced with a confusing array of oral hygiene products making a wide variety of either therapeutic or cosmetic claims. For many patients, mechanical oral hygiene measures alone may be insufficient to achieve the plaque removal necessary for gingival health or the perception of a clean mouth and fresh breath. Mouthrinses are very commonly used adjunctively to support mechanical measures and present an area of confusion for both dental professionals and patients. A new anti-plaque mouthrinse with a novel mechanism of action will soon be available in the United States. This review presents the rationale for the use of mouthrinses and compares and contrasts therapeutic agents, including this new mouthrinse, for reduction in plaque and gingivitis.
Introduction
The prevention and control of gingivitis and periodontitis are challenges for the dental professional in the everyday improvement and maintenance of oral health for their patients. Less than 20% of all gingivitis cases will advance to periodontitis and the identification of those who will progress is still impossible to determine, as there is currently no method to identify patients who are vulnerable to this progression.1 Good oral hygiene, through adequate plaque control and management of gingival inflammation, are fundamental to oral health.
Gingivitis and periodontitis are widespread diseases, and prevention is dependent on the control of supragingival plaque. The experimental gingivitis studies of Löe and colleagues clearly illustrated the action of plaque accumulation in the development of gingivitis.2 When accumulated plaque was removed through normal oral hygiene, gingival inflammation subsided, validating the significance of plaque as the primary etiological factor in gingival inflammation.
Toothbrushing with toothpaste is the most frequently employed method for basic oral hygiene in the United States, although very few patients are able to attain ideal plaque removal through this approach alone. Flossing and interproximal brushing are essential factors in decreasing plaque levels further. While patients who employ these classic oral hygiene measures may exhibit less gingivitis and periodontitis, areas of plaque accumulation may still remain especially in the dento-gingival areas. These unreachable areas require additional efforts if gingival inflammation is to be avoided. Mouthrinses are easy to use and by the simple action of rinsing are distributed widely throughout the oral cavity, accessing not only areas inadequately treated by other oral hygiene methods but also soft tissue areas with significant bacterial reservoirs. The adjunctive use of anti-plaque mouthrinses can provide significant benefits to those patients who are unable to maintain the highest levels of mechanical cleaning. Mouthrinses are well accepted today and have been used for thousands of years, dating back to 2700 B.C. in Chinese medicine.3
Plaque: Challenges to the Elimination of Gingival Inflammation
Gingival inflammation, and to some degree periodontitis, is still highly prevalent despite improvements in oral hygiene.4 Clinical experiences of dentists and dental hygienists are mirrored by epidemiologic data from the United States and other countries, revealing the presence of gingivitis in a majority of people. As the largest study in the United States, the Third National Health and Nutrition Examination Survey revealed that 63% of examined dentate people in the United States had gingivitis.5 The prevalence of gingivitis was reported to be 50.3% in all people between the ages of 30 and 90 years, with a mean of 13.5% of teeth involved. There is a strong correlation between supragingival plaque levels and chronic gingivitis.6 Subgingival plaque, derived from supragingival plaque, is also closely associated with the advance of chronic periodontal diseases. Therefore, the control of supragingival plaque is primary to the prevention of periodontal diseases.7,8 The degree of difficulty in achieving high levels of plaque removal was clearly demonstrated in a study of adults in which the researchers found that no individual was entirely plaque-free. Visible plaque on more than 90% of tooth surfaces was found in 35.7% of study participants.9
From clinical experience and population-based studies, it is clear that while many people do use mechanical plaque-control methods, they do not use those methods at levels effective enough to maintain adequate oral hygiene. The need for additional help in controlling bacterial plaque provides justification for the use of anti-plaque mouthrinses with clinically proven anti-gingivitis effectiveness as adjuncts to mechanical oral hygiene procedures.3
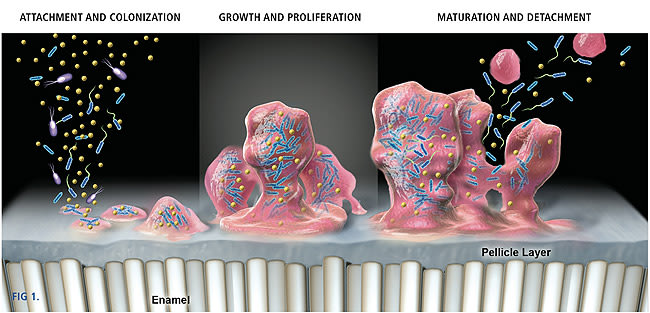
Many bacterial species can adhere to both dental and mucosal surfaces. Following initial attachment, the bacteria multiply and form micro-colonies. Left undisturbed, these micro-colonies grow and become confluent, forming a biofilm in which microorganisms are intimately associated with each other in a matrix of exopolymers of bacterial and salivary origin.10 Dental plaque exists as a complex biofilm in which many species of bacteria co-exist in highly organized and structured communities. This enables an ecosystem where different communities are not only dependent upon each other, but have signaling pathways of communication. The protective matrix is difficult to penetrate and, therefore, the susceptibility of microbes to antimicrobial agents is much reduced in biofilms.11 The effect of antimicrobial agents in established biofilms is limited to the superficial layers. It has been shown that bacterial microcosms are able to recover rapidly after exposure to chlorhexidine.12 The bacteria in biofilms undergo phenotypic changes that may render the bacteria more resistant. Figure 1 illustrates the biofilm formation on a tooth surface. An aggressive biofilm will progress from the supragingival to the subgingival region, ultimately leading to gingivitis or periodontitis.
Reservoirs of pathogenic bacteria exist within the oral mucosa, especially on the surface of the tongue and around the tonsils. Continual streams of bacteria may be seeded from these reservoirs and either transferred by direct physical contact to the tooth surface or via saliva. Classic mechanical oral hygiene is directed toward the dental and gingival areas, which constitute only about 20% of the available surfaces of the oral cavity. Even if this 20% were cleaned immaculately, the remaining 80% forms an ecosystem maintaining a ready source of necessary organisms for the rapid recolonization of the recently cleaned hard surfaces. The challenge of achieving a clean dento-gingival complex is almost impossible without addressing the ecosystem of recolonization. Supplementing mechanical plaque control methods with effective anti-plaque mouthrinses is a way of countering this bacterial advance by reaching mucosal sites unaffected by mechanical plaque control methods. Studies have shown the effectiveness of employing an antimicrobial mouthrinse that significantly reduces both salivary13 and mucosal14 levels of bacteria. Adding an anti-plaque mouthrinse to daily oral hygiene regimens will help to reduce the total oral bacterial burden.
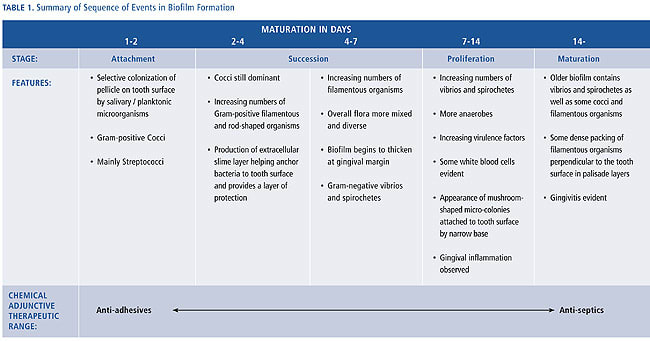
Gingivitis occurs when supragingival plaque reaches quantitative and qualitative levels of bacterial complexity that compromise gingival health. Although the microbiology of gingivitis is not yet fully understood, the sequencing of plaque formation indicates how interventions may prevent the development of gingivitis. Opportunities exist at any of these critical stages: bacterial attachment, bacterial succession and proliferation, and maturation to disrupt and inhibit the plaque mass. Although mechanical oral hygiene addresses all of these stages, the use of chemical agents enables more objective targeting.
Helping the Patient: Overcoming the Challenges of Plaque
Today, patients are acutely aware and place a great deal of importance on oral health. The perception of clean teeth and fresh breath provides an individual with positive feedback regarding the health of the oral cavity. It is also important for social and emotional reasons and, therefore, general well-being. In many cases frequent oral hygiene is implemented as one element of daily grooming, rather than for the prevention or avoidance of disease.
Despite this knowledge, many individuals may not realize that even when brushing for 2 minutes, only about half of the plaque is removed from their teeth.15 This apparently occurs because certain tooth surfaces receive little or no attention during brushing.16 In considering this further, few individuals brush for more than 60 seconds. Therefore, the adjunctive use of antimicrobials provides a way of overcoming deficiencies in the mechanical tooth-cleaning habits practiced by many individuals.
For those few people who can achieve very high levels of plaque removal, tooth cleaning only once every 2 days has been shown to prevent gingivitis.17 Presumably, the plaque biofilm never reaches any level of maturation during this time. However, the professional recommendation is to brush twice a day, which is evidenced as a benefit to gingival health.18 Poor or inadequate oral hygiene leads to excessive plaque build-up and maturation within the oral cavity, which may subsequently result in gingival inflammation and periodontal disease.
While it is possible to maintain a level of oral hygiene sufficient to control gingivitis using mechanical methods alone, the majority of people are unable to achieve this control on a consistent basis. Mouthrinses provide an additional level of effective care adjunctive to tooth and interproximal cleaning, and prophylaxis. In support of this, the rationale for daily use of anti-plaque mouthrinses is two-fold:
- As a component added adjunctively to mechanical oral hygiene regimens for the control and prevention of periodontal diseases.
- As a method of delivering anti-plaque agents to mucosal sites throughout the mouth that harbor pathogenic bacteria capable of recolonizing supragingival and subgingival tooth surfaces.1
Long-term effects of treatment for periodontal disease patients reveal that success depends on maintaining plaque levels that are consistent with gingival health.19 Soon after dental prophylaxis, bacteria start to recolonize the tooth surface and plaque build-up begins again. Bacteria diffuse from mucosal and tooth surfaces into saliva and colonize other areas of the mouth. Unstimulated saliva contains 50 million to 100 million bacteria per milliliter,20,21 so the oral surfaces are constantly immersed in a reservoir of microorganisms.22
Advances in our knowledge of oral microbial ecology have enhanced our understanding of the role that antimicrobial mouthrinses can play in controlling plaque biofilm and related periodontal diseases. Supragingival and subgingival tooth surfaces are part of a larger ecological system that includes oral mucosal surfaces and saliva.23 Studies comparing the bacterial composition of supragingival and subgingival plaque with that of saliva and various mucosal surfaces have indicated that the mucosa serves as a source of bacteria. The mucosa is the origin of pathogens that may readily recolonize teeth after a dental prophylaxis or periodontal therapy.23 Mechanical plaque control methods are limited in their reach and have little effect upon reservoirs of microbes from the larger areas of oral mucosal tissue.
One of the preferred methods of controlling supragingival plaque and preventing gingivitis is through the use of chemical agents. The active agents should prevent biofilm formation without affecting the microbial balance and ecosystem within the oral cavity, avoiding an overgrowth by resistant organisms. Depending upon the goals of the preventive measures, various strategies may be considered. Anti-plaque agents with properties other then bactericidal or bacteriostatic properties, such as anti-adhesive agents, may be primarily used to keep plaque from developing on a clean tooth surface. True antimicrobial agents may be more appropriately used as a secondary prevention method to restore and achieve oral health. Dental professionals must be attentive to patient proficiency in plaque removal, and reinforce that using a mouthrinse is not a substitute for mechanical oral hygiene procedures, but complementary and supportive to patient mechanical efforts.
Agents in Therapeutic Mouthrinses
Most currently available chemical plaque control and anti-gingivitis agents have been formulated in mouthrinse delivery systems. There are many oral hygiene products that function as delivery devices for plaque control and anti-gingivitis agents. Of all these, toothpaste may be considered the ideal vehicle, by virtue of widespread use and twice-daily application. However, its complex formulation of abrasives, thickeners, detergents, sweeteners, humectants, flavoring agents, and other active agents such as sodium fluoride (caries prevention) and polyphosphates (whitening or tartar control), presents a challenge. The issue is to keep the active agent for plaque control from reacting with the other ingredients and compromising the effectiveness of the toothpaste itself. Several detergents, such as sodium lauryl sulfate, are anionic (negatively charged), whereas many commonly used antiseptic agents are cationic (positively charged). They may compromise each other’s activity when combined. As toothpaste is diluted with saliva, a foam slurry develops and is dispersed within the oral cavity. A toothbrush and toothpaste may not effectively reach all areas of the mouth, especially where many microorganisms reside on the soft tissues and the interproximal surfaces between teeth. In reality, the act of toothbrushing is selectively restricted to dental hard surfaces.
Mouthrinses are the most common vehicle for chemical plaque-control and anti-gingivitis agents, and are typically less complex than toothpastes in their formulation and ingredients. Most often, the formulations are aqueous solutions composed of flavoring agents, colors, and preservatives as well as an active agent and, perhaps, a solubilizing agent, such as denatured ethyl alcohol. Recently, formulations that previously relied on alcohol for solubilization have undergone reformulation and are now presented in either low- or no-alcohol variants. The presence or absence of alcohol does not necessarily indicate whether a specific rinse formulation has therapeutic activity or is merely cosmetic.
Mouthrinses are dispersed easily throughout the oral cavity using a swishing action that reaches not only dental hard surfaces but most soft tissue areas as well. As a result, the action of mouthrinses extends to areas of microbial deposition, such as the dorsum of the tongue, that may act as a reservoir for recolonization of the dental plaque biofilm.24
Many oral rinses are available in the marketplace today. The shopper is presented with a wide, perhaps confusing, choice of products with many differing claims. Therefore, it behooves the dental professional to be knowledgeable and up to date regarding the availability, effectiveness, and differences of various oral rinse products. Patients routinely seek advice about oral hygiene products—and expect an informed review—from their dental professional. For many patients, a recommendation for a specific agent will be necessary to augment their mechanical oral hygiene efforts. Compliance with any recommendations will be necessary to improve gingival health.
Currently marketed mouthrinses are available either over-the-counter (OTC) or by prescription only. In OTC therapeutic rinses, there are currently three classes of active agents:
- amine alcohols
- a mixture of essential oils
- quaternary ammonium compounds
Many OTC products containing these active ingredients have proven therapeutic benefits that reduce levels of both plaque and gingivitis. In order to make these therapeutic claims, the Food and Drug Administration requires randomized controlled clinical trials. In the prescription-only category, chlorhexidine is currently the only manufactured formulation and is available as a 0.12% oral rinse. Formulations that do not fall into either of the therapeutic categories are cosmetic in nature. "Cosmetic" oral rinses do not reduce plaque or gingivitis but may claim to act as mouth or breath fresheners. These cosmetic claims are not supported by clinical evidence.
Click to view Table 1 full size.
Bisbiguanide Antiseptics—Chlorhexidine
Chlorhexidine digluconate 0.12% is the only formulation of this group of antiseptics currently available as a mouthrinse in the United States although different concentrations (0.06% to 0.2%) are available in other countries. Originally, this prescription-only mouthrinse was formulated with alcohol (approximately 11.6%) but now alcohol-free formulations are available. Alcohol has been used in mouthrinses as an emulsifier or solubilizing agent and as an astringent. However, it is this astringency that may compromise patient compliance due to stinging, burning, or mucosal pain, which have been demonstrated to be dose-dependent.25 Ethanol has been shown to produce surface softening of dental resins and composite materials leading to increased wear rates.26 Alcohol-containing formulations may be undesirable for patients undergoing treatment for alcoholism, pregnant or nursing women, diabetics, or those who choose to avoid alcohol for cultural or religious needs or considerations. Therefore, the recommendation for alcohol-free formulations may enhance patient compliance for a wide range of reasons.
Chlorhexidine has been widely used in medicine and surgery for presurgical disinfection since the 1940s and was first investigated for effectiveness in the oral cavity in 1970.27 The chlorhexidine molecule is a strong base, with two positive charges (dicationic) at pH levels above 3.5.28 These two positive charges make chlorhexidine extremely interactive with anions and are the basis of its clinical effectiveness as well as its unwanted effects.
Chlorhexidine has a broad range of antimicrobial activity that includes Gram-positive and Gram-negative bacteria; some fungi and yeasts, including Candida; and some viruses, including human immunodeficiency virus (HIV). Bacterial resistance after long-term use has not been reported. The antibacterial mode of action is thought to occur against the cell wall. Bacterial cell walls are characteristically negatively charged. The positively charged chlorhexidine molecule is rapidly attracted to the negatively charged cell surface. The integrity of the cell membrane is altered at lower concentrations, leading to increased permeability and leakage of intracellular, low molecular weight contents. This stage is considered bacteriostatic and is reversible so the cell can recover. At higher concentrations, gross bactericidal damage will occur. This is reflected by reduced leakage of the low molecular weight contents and coagulation along with precipitation of the cytoplasm, which is irreversible and, therefore, bactericidal.29
Chlorhexidine also binds via adsorption to the different surfaces in the oral cavity, as well as to the pellicle and saliva. This effect has been postulated to provide several benefits that may help explain its persistence in the oral cavity (substantivity) and its mode of action. As chlorhexidine binds to surfaces, a reservoir is formed. Gradual desorption allows more chlorhexidine to be transported by saliva to the tooth surface and the bacterial cell wall.30 In binding directly with the tooth surface, the chlorhexidine molecule occupies sites that could be used by salivary proteins and bacteria to bind to the tooth. This action by chlorhexidine may inhibit the early stages of plaque formation. Depending on the concentration of chlorhexidine, the bactericidal or bacteriostatic effects will compromise bacteria attaching to the tooth surface. In this mechanism, chlorhexidine may be more effective as a plaque preventive agent, rather than a plaque removal agent.31
This tooth surface activity may also explain the unwanted effect of staining. Addy has explained staining in terms of a local precipitation reaction between chromogens found in foods and beverages and the tooth-bound chlorhexidine. Avoidance of these foods and beverages during treatment with chlorhexidine, especially soon after its application, should reduce the degree of stain formation.32 Other unwanted effects reported33 include taste perturbation (to salt), mucosal erosion (rare at the 0.12% concentration), enhanced supragingival calculus formation, a tingling sensation of the tongue, and anesthesia-like effects. For these reasons, chlorhexidine rinses are prescribed only for a relatively short duration to help control acute or severe inflammatory conditions, resulting in plaque reduction and improved gingival health. Chlorhexidine is generally not indicated for long-term maintenance due to staining and calculus formation.
Chlorhexidine has been extensively studied in clinical trials and has been referred to as the gold standard.34 Clinical trials have demonstrated typical reductions in plaque of 21.6% to 60.9% and gingivitis of 18.2% to 42.5%.35,36,55
Click to view Table 2 full size.
Phenols—Essential Oils
Phenol-related essential oils have been used in mouthrinses for many years. Listerine®, a formulation derived from Lister’s original work with carbolic acid, is a mouthrinse with a very long history dating back to the 19th century. The rinse contains two phenol-related essential oils, thymol (0.064%) and eucalyptol (0.092%) mixed with menthol (0.042%) and methyl salicylate (0.060%). This fixed combination of essential oils is dispersed in a denatured alcohol vehicle (between 21.6% to 26.9%), although reduced alcohol formulations are now available. All mouthrinses containing alcohol may be limited to the populations that can tolerate the local side effects; however, Listerine has been used by millions of consumers since its introduction more than 100 years ago and remains one of the most commonly available and used OTC therapeutic mouthrinses today.37
The mechanisms of action of this formulation against bacteria are complex. At high concentrations, there is a disruption of the cell wall and precipitation of cell proteins; essential enzymes are inhibited at lower concentrations. This formulation of essential oils can penetrate the plaque biofilm and exert bactericidal activity. The bacterial load is reduced with a concomitant decrease in plaque mass and pathogenicity.38
A number of long-term studies have been conducted on this rinse of a fixed combination of essential oils that have shown consistent adjunctive benefits. Clinical trials have demonstrated plaque reductions of 13.8% to 56.3%, and gingivitis reductions of 14% to 35.9%.36,39-43,55 A study involving flossing and brushing with the mouthrinse showed an additional 15.8% reduction in interproximal gingivitis compared to a reduction of only 7.7% in the flossing and brushing group not using the mouthrinse.43
Quaternary Ammonium Compounds—Cetylpyridinium Chloride
Cetylpyridinium chloride (CPC) is a cationic surface-active agent that has a broad antimicrobial spectrum of activity, involving the rapid destruction of Gram-positive pathogens and yeasts. The mode of action against bacteria is through the disruption of the membrane function, the leakage of cytoplasmic material, and, ultimately, the collapse of the intracellular equilibrium. CPC is found in many mouthrinses, including those with a therapeutic benefit and also those with only cosmetic claims. Clinical research has shown CPC mouthrinses to have anti-plaque activity when used alone or in combination with toothbrushing. A recent meta-analysis from a systematic review supported the plaque- and gingivitis-inhibiting effect of CPC-containing mouthrinses. It concluded that CPC rinses, when used as adjuncts to oral hygiene, provide a small but significant additional benefit in reducing both plaque accumulation and gingival inflammation.44
However, not all formulations of CPC-containing mouthrinses provide the same degree of clinical benefit due to the bioavailability of the CPC. The formulation of the vehicle ingredients, solubilizers, preservatives, stabilizers, coloring agents, etc, and the way they are combined together can have a significant impact on the bioavailability of the CPC. Increased bioavailability is associated with higher probability of effectiveness, greater anti-plaque activity and greater reductions in gingivitis. Decreased bioavailability and lower concentrations are commonly associated with cosmetic claims alone such as in-vitro germ killing, fresh breath, etc. The FDA Plaque subcommittee deemed CPC to be safe and effective for the treatment of plaque-induced gingivitis within a concentration range of 0.045% to 0.10% when present in a high-bioavailable matrix.21 The substantivity of CPC is reported to be between 3 to 5 hours, due at least in part to its cationic nature. Staining caused by CPC has a similar dietary etiology to chlorhexidine solutions but appears to be less severe.45
In clinical trials, CPC rinses have shown reductions in plaque and gingivitis. A mouthrinse containing 0.07% high bioavailable CPC in an alcohol-free formulation (Pro-Health®) demonstrated a 15.8% reduction in plaque, 15.4% reduction in gingival inflammation, and 33.3% less gingival bleeding, relative to a placebo group after 6 months.46
Amine Alcohols—Delmopinol Hydrochloride
First- and second-generation versions of anti-plaque and anti-gingivitis agents have been on the market in the United States for many years. Their mechanism of action is the inhibition or killing of microbes of the oral flora. The next generation of agents has begun to emerge that has a unique ability to inhibit or disrupt the formation of plaque, while possessing little if any effect on the bacteria, avoiding disruption to the balance of bacterial flora found in a healthy mouth. Delmopinol hydrochloride (a morpholinoethanol derivative) is an amine alcohol that is relatively new to oral hygiene products, and functions as a surface-active agent with low antimicrobial potency.47 Delmopinol has also been shown to have the ability to interact with pellicle constituents and inhibit glucan synthesis by Streptococcus mutans.48,49 Delmopinol has little or no demonstrable effect on the bacteria, but it interferes with plaque/biofilm matrix formation. It reduces the adherence of primary plaque-forming bacteria in the development of plaque biofilm. Studies have shown that the nascent biofilm mass is loosely adherent and that there is a significant reduction in the proportion of dextran producing cocci.50 The interference with plaque matrix formation leads to the plaque deposit being loosely adherent and possibly easier to remove.
Delmopinol hydrochloride 0.2% mouthrinse has been shown to be effective against plaque and gingivitis in both short-term no-oral-hygiene studies and long-term home-use studies. Arguably, the short-term no-oral-hygiene studies showed plaque inhibition closer to that of chlorhexidine than any other previous agent.51
Although recently approved in the United States, delmopinol hydrochloride 0.2% mouthrinses have undergone rigorous clinical testing in no fewer than 29 clinical studies. The first ever meta-analysis of mouthrinse efficacy data based upon eight of these delmopinol studies was published in 2007.52 The conclusion of the meta-analysis was that this third-generation agent was effective as an adjunctive measure for reducing plaque burden and gingivitis, whether or not used under supervision. It was also noted that delmopinol 0.2% met the efficacy criteria of the American Dental Association for anti-plaque and anti-gingivitis mouthrinses.
In clinical trials, delmopinol rinses have demonstrated plaque reductions of 9.3% to 35%, bleeding on probing reductions of 18% to 36%, and gingivitis reductions of up to 18%.51,53-54,57 A meta-analysis of eight double-blind, parallel group studies conducted by seven different independent research groups supported the effectiveness of delmopinol 0.2% mouthrinse as an effective measure for reducing plaque and gingivitis.52
Managing the Needs of the Patient
Patients seldom achieve the expectations of dentists and dental hygienists about plaque removal. Few patients conducting mechanical-only plaque control (toothbrushing and interproximal cleaning) are able to achieve adequate and widespread levels of plaque removal consistent with gingival health. Periodontal diseases are plaque-induced and are initiated by a host-inflammatory response to the maturing plaque biofilm. Plaque is the primary etiological factor for gingival inflammation. Considering that plaque-induced gingivitis always precedes the occurrence and re-occurrence of periodontitis, the prevention of periodontal diseases depends upon the control of supragingival plaque.
Opportunities exist to broaden the armamentarium of plaque control using various chemical agents, which act through various strategies:
- interference with the initial adhesion of the oral bacteria
- disruption of the co-aggregation mechanisms
- suppression of the multiplication or direct killing of micro-organisms through bacteriostatic or bactericidal means
These agents may be delivered in various forms: toothpastes, gels, sprays, or mouthrinses. Mouthrinsing is a well-accepted cosmetic and therapeutic activity that delivers active ingredients throughout the oral cavity, reaching areas typically inaccessible by mechanical oral hygiene methods alone. Mechanical oral hygiene is targeted only toward the tooth surfaces and the dento-gingival margins (approximately 20% of the total oral surface area), neglecting the mucosal surfaces in the single ecosystem of the oral cavity.
Today in the United States, there are four categories of anti-plaque agents available in mouthrinses: chlorhexidine, delmopinol, cetylpyridinium chloride, and a fixed combination of essential oils.
Chlorhexidine, often referred to as the gold standard, is available by prescription only. It is highly effective when used adjunctively to in-office and at-home procedures for gaining control of periodontal diseases. It is also prescribed following in-office soft tissue procedures when mechanical plaque control alone may be insufficient for good hygiene. In such cases, the adjunctive use of chlorhexidine may help control plaque until regular mechanical cleaning can be reinstituted. Chlorhexidine is ideal to help control plaque for a relatively short period during active treatment. With prolonged use, the unwanted effects of staining and calculus build-up become apparent. These, together with its bitter taste and burning sensation from its alcohol content, tend to compromise patient compliance and successful outcomes of treatment. Because alcohol is of no therapeutic value, alcohol-free formulations of chlorhexidine mouthrinse (Sunstar G·U·M®) are now available and should be routinely prescribed.
As gingival health is re-established, what can the patient do to maintain gingival health and support their less-than-optimal mechanical oral hygiene? In determining recommendations, many factors should be considered, the most important being patient compliance. It is critical that a patient adopts the advocated behavior. Approved therapeutic mouthrinses have clinically demonstrated benefits, so it is essential to recommend or endorse the product that the patient is most likely to use. The recommendation is primarily based upon sensory perceptions and should be balanced with the clinical need.
G·U·M® PerioShield™ Oral Health Rinse (Delmopinol, 0.2%), about to be introduced in the United States as a surface-active anti-plaque agent, interferes with plaque formation by disrupting the matrix and thus enabling easy removal. This approach is relatively new and does not rely upon killing or inhibiting the microbiota. Delmopinol disrupts the development and maturation of the established and harmful biofilm while maintaining the biological equilibrium within the oral cavity. Given its inferred properties of keeping a clean tooth clean, the concept of using delmopinol for long-term daily maintenance after active treatment with chlorhexidine is attractive and may help prevent relapse in the many patients whose mechanical cleaning is not conducive to the maintenance of ongoing gingival health. Delmopinol has very few antimicrobial properties and, as its mechanism of action inhibits the development and maturation of the plaque biofilm, it is distinctly attractive for patients concerned about antimicrobial resistance, overgrowths of resistant strains, or maintaining equilibrium of the oral ecosystem.
The formulation of a fixed combination of essential oils has stood the test of time in the consumer marketplace and also provides antimicrobial benefits. Many variants are now available, with even lower concentrations of alcohol and milder flavors.
Cetylpyridinium chloride is available in many formulations. For therapeutic benefit, care must be taken in the selection of the formula to ensure a high degree of bioavailability of the active agent and, therefore, the ability to inhibit plaque and reduce gingival inflammation. Crest® Pro Health® is a relatively new formulation containing bioavailable CPC in an alcohol-free presentation. CPC is a cationically charged molecule that, in some cases, may be associated with a degree of stain after prolonged use.
Conclusion
Within the population, few patients will ever achieve the degree of plaque control necessary for gingival health by mechanical means. This paper has outlined the benefits of adding chemical agents adjunctively to daily oral hygiene regimens to achieve greater reductions of both plaque and gingival inflammation.
Key considerations are:
- Adjunctive use of any of the therapeutic oral rinses above can provide significant benefits to the many patients who cannot maintain adequate levels of plaque and gingivitis control through mechanical means alone.
- It is imperative that patients understand that the use of an anti-plaque/anti-gingivitis agent is not the silver bullet of oral hygiene.
- The key to oral health begins with a well-designed toothbrush and toothpaste that encourages individual patient compliance and adherence to the technique recommended by the dental professional.
- Adjunctive agents for plaque and gingivitis control will nearly always be necessary.
- An individual approach to the factors determining a patient’s gingival condition is warranted.
Therapeutic mouthrinses, with their ability to reach beyond mechanical plaque control, have a very important role in the reduction of dental plaque and gingivitis.
References
1. Barnett ML. The rationale for the daily use of an antimicrobial mouthrinse. J Am Dent Assoc. 2006;137(suppl):16S-21S.
2. Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177-87.
3. Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988;15(8):488-498.
4. Baehni PC, Bourgeois DM. Epidemiology of periodontal health and disease. In: Lang NP, Attstrom R, Löe H, eds. Proceedings of the European Workshop on Mechanical Plaque Control. Berlin: Quintessence Publishing Company; 1998:19-34.
5. Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70(1):30-43.
6. Ash M, Gitlin BN, Smith NA. Correlation between plaque and gingivitis. J Periodontol. 1964;35:425-429.
7. World Workshop in Periodontics. Consensus report on prevention. Ann Periodontol. 1996a:250-255.
8. Hancock EB, Prevention. World Workshop in Periodontics. Ann Periodontol. 1996(1):223-249.
9. Christersson LA, Grossi SG, Dunford RG, et al. Dental plaque and calculus: risk indicators for their formation. J Dent Res. 1992;71(7):1425-1430.
10. Listgarten MA. Formation of dental plaque and other oral biofilms. In: Newman HN, Wilson M, eds. Dental Plaque Revisited: Oral Biofilms in Health and Disease. Cardiff: BioLine; 1999:187-210.
11. Larsen T, Fiehn NE. Resistance of Streptococcus sanguis biofilms to antimicrobial agents. APMIS. 1996;104(4):280-284.
12. Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45(4):999-1007.
13. DePaola LG, Minah GE, Overholser CD, et al. Effect of an antiseptic mouthrinse on salivary microbiota. Am J Dent. 1996;9(3):93-95.
14. Fine DH, Furgang D, Sinatra K, et al. In vivo antimicrobial effectiveness of an essential oil-containing mouth rinse 12 h after a single use and 14 days’ use. J Clin Periodontol. 2005;32(4):335-340.
15. De la Rosa MR, Zacarias GJ, Johnston DA, Radike AW. Plaque growth and removal with daily toothbrushing. J Periodontol. 1979;50(12):661-664.
16. MacGregor ID, Rugg-Gunn AJ. A survey of toothbrushing sequence in children and young adults. J Periodontal Res. 1979;14(3)225-230.
17. McNabb H, Mombelli A, Lang NP. Supragingival cleaning 3 times a week. The microbiological effects in moderately deep pockets. J Clin Periodontol. 1992;19(5):348-356.
18. Frandsen A. Mechanical oral hygiene practices In: Löe H, Kleinman DV, eds. Dental Plaque Control Measures and Oral Hygiene Practices. 1st ed. Oxford: IRL Press; 1986:93-116.
19. Axelsson P, Lindhe J. The significance of maintenance care in the treatment of periodontal disease. J Clin Periodontol. 1981;8(4):281-294.
20. Imrey PB, Chilton NW, Pihlstrom BL, et al. Recommended revisions to American Dental Association guidelines for acceptance of chemotherapeutic products for gingivitis control. Report of the Task Force on Design and Analysis in Dental and Oral Research to the Council on Therapeutics of the American Dental Association. J Periodontal Res. 1994;29(4):299-304.
21. U.S. Food and Drug Administration. Oral health care drug products for over-the-counter human use; antigingivitis/antiplaque drug products; establishment of a monograph; proposed rules. Fed Regist. 2003;68:32232-32287.
22. Liljemark WF, Bloomquist CG, Reilly BE, et al. Growth dynamics in a natural biofilm and its impact on oral disease management. Adv Dent Res. 1997;11(1):14-23.
23. Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30(7):644-654.
24. Pitts G, Pianotti R, Feary TW, et al. The in vivo effects of an antiseptic mouthwash on odor-producing microorganisms. J Dent Res. 1981;60(11):1891-1896.
25. Bolanowski SJ, Gesheider GA, Sutton SV. Relationship between oral pain and ethanol concentration in mouthrinses. J Periodontal Res. 1995;30(3):192-197.
26. Penugonda B, Setttembrini L, Scherer W, et al. Alcohol-containing mouthwashes: effect on composite hardness. J Clin Dent. 1994;5(2):60-62.
27. Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5(2):79-83.
28. Denton GW. Chlorhexidine. In: Block SS, ed. Disinfection, Sterilization and Preservation. 4th ed. Philadelphia, PA: Lea and Febiger; 1991:274-289.
29. Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55-62.
30. Gjermo P, Bonesvoll P, Rölla G. Relationship between plaque-inhibiting effect and retention of chlorhexidine in the human oral cavity. Arch Oral Biol. 1974:19(11):1031-1034.
31. Jenkins S, Addy M, Wade W. The mechanism of action of chlorhexidine. A study of plaque growth on enamel inserts in vivo. J Clin Periodontol. 1988:15(7):415-424.
32. Addy M, Moran J. Chemical supragingival plaque control. In: Lindhe J, Lang NP, Karring T, eds. Clinical Periodontology and Implant Dentistry. 5th ed. Oxford: Blackwell Publishing, Ltd.; 2008:734-765.
33. Flotra L, Gjermo P, Rolla G, Waerhaug J. Side effects of chlorhexidine mouthwashes. Scandinavian Journal of Dental Research. 1971;79:119-125.
34. Jones CG. Chlorhexidine: is it still the gold standard? In: Addy M, Moran JM, eds. Toothpaste, mouth rinse and other topical remedies in periodontics. Periodontol 2000. 1997;15:55-62.
35. Grossman E, Reiter G, Sturzenberger OP, et al. Six-month study of the effects of a chlorhexidine mouthrinse on gingivitis in adults. J Perio Res. 1986;21(suppl):33-43.
36. Overholser CD, Meiller TF, DePaola LG, et al. Comparative effects of 2 chemotherapeutic mouthrinses on the development of supragingival dental plaque and gingivitis. J Clin Periodontol. 1990;17(8):575-579.
37. Axelsson P. Preventive Materials, Methods and Programs. Vol. 4. Surrey, UK: Quintessence; 2004:184.
38. Fine DH, Letizia J, Mandel ID. The effect of rinsing with Listerine antiseptic on the properties of developing dental plaque. J Clin Periodontol. 1985;12(8):660-666.
39. Lamster IB, Alfano MC, Seiger MC, Gordon JM. The effect of Listerine Antiseptic on reduction of existing plaque and gingivitis. Clin Prev Dent. 1983;5:12-16.
40. Gordon JM, Lamster IB, Sieger MC. Efficacy of Listerine antiseptic in inhibiting the development of plaque and gingivitis. J Clin Periodontol. 1985;12(8):697-704.
41. DePaola LG, Overholser CD, Meiller TF, et al. Chemotherapeutic inhibition of supragingival dental plaque and gingivitis development. J Clin Periodontol. 1989;16(5):311-315.
42. Charles CH, Sharma NC, Galustians HJ, et al. Comparative efficacy of an antiseptic mouthrinse and an antiplaque/antigingivitis dentifrice. A six-month clinical trial. J Am Dent Assoc. 2001;132(5):670-675.
43. Sharma N, Charles CH, Lynch MC, et al. Adjunctive benefit of an essential oil-containing mouthrinse in reducing plaque and gingivitis in patients who brush and floss regularly: a six-month study. J Am Dent Assoc. 2004;135(4):496-504.
44. Haps S, Slot DE, Berchier CE, Van der Weijden GA. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6(4):290-303.
45. Roberts WR, Addy M. Comparison of the in vivo and in vitro antibacterial properties of antiseptic mouthrinses containing chlorhexidine, alexidine, cetyl pyridinium chloride and hexetidine. Relevance to mode of action. J Clin Periodontol. 1981;8(4):295-310.
46. Mankodi S, Bauroth K, Witt JJ, et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent. 2005;18(Spec No. 9):9A-14A.
47. Simonsson T, Hvid EB, Rundegren J, Edwardsson S. Effect of delmopinol on in vitro dental plaque formation, bacterial acid production and the number of microorganisms in human saliva. Oral Microbiol Immunol. 1991;6(5):305-309.
48. Rundegren J, Simonsson T, Petersson L, et al. Effect of delmopinol on the cohesion of glucan-containing plaque formed by Streptococcus mutans in a flow cell system. J Dent Res. 1992;71(11):1792-1796.
49. Steinberg D, Beeman D, Bowen WH. Interactions of delmopinol with constituents of experimental pellicle. J Dent Res. 1992;71(11):1797-1802.
50. Elworthy AJ, Edgar R, Moran J, et al. A 6-month home-usage trial of 0.1% and 0.2% delmopinol mouthwashes (II). Effects on the plaque microflora. J Clin Periodontol. 1995;22(7):527-532.
51. Claydon N, Hunter L, Moran J, et al. A 6-month home-usage trial of 0.1% and 0.2% delmopinol mouthwashes (I). Effects on plaque, gingivitis, supragingival calculus and tooth staining. J Clin Periodontol. 1996;23(3 Pt 1):220-228.
52. Addy M, Moran J, Newcombe RG. Meta-analyses of studies of 0.2% delmopinol mouth rinse as an adjunct to gingival health and plaque control measures. J Clin Periodontol. 2007;34(1)58-65. Epub 2006.
53. Lang NP, Hase JC, Grassi M, et al. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis. 1998;4(2):105-113.
54. Hase JC, Attstrom R, Edwardsson S, et al. 6-month use of 0.2% delmopinol hydrochloride in comparison with 0.2% chlorhexidine digluconate and placebo. (I). Effect on plaque formation and gingivitis. J Clin Periodontol. 1998;25(9):746-753.
55. Charles CH, Mostler KM, Bartels LL, Mankodi SM. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J Clin Periodontol. 2004;31(10):878-884.
56. Charles CH, Sharma NC, Galustians HJ, et al. Comparative efficacy of an antiseptic mouthrinse and an antiplaque/antigingivitis dentifrice. A six-month clinical trial. J Am Dent Assoc. 2001:132(5):670-675.
57. Lang NP, Hase JC, Grassi M, et al. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis. 1998;4(2):105-113.
Disclosure
This Whitepaper is supported by an unrestricted grant from Sunstar.