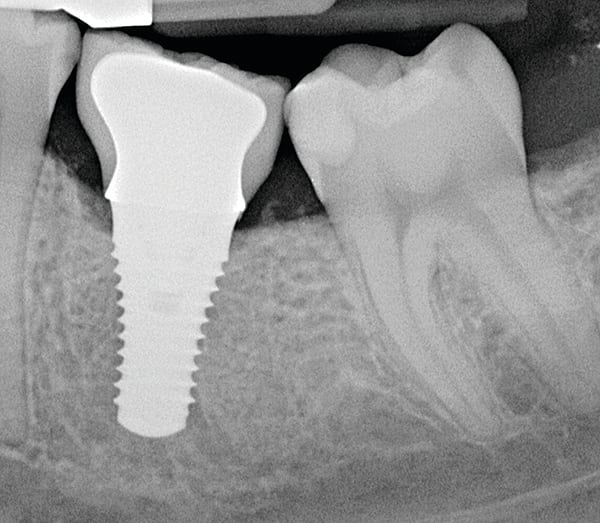
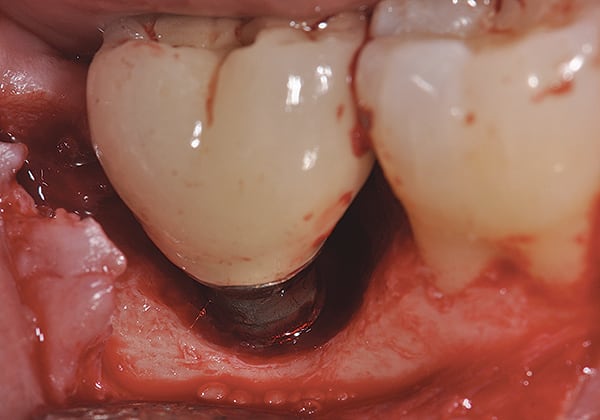
abstract
Given that an increasing number of patients—including those with risk factors for complications—are insisting on implants, it is important for general practitioners and specialists to work together to improve the predictability for a successful outcome. This involves making a greater effort to prevent peri-implant diseases—both peri-implantitis and peri-implant mucositis—through proper hygiene and adherence to regular maintenance care. Moreover, in those situations where problems arise, it is imperative to recognize and render treatment at the earliest stage possible. This requires careful record keeping—ie, radiographs taken at the time of implant placement, restoration, and at the first signs of disease that could signal bone loss; the amount of bleeding on probing (BOP); and increased probing depths—which should be evaluated at every visit. Appropriate co-management by the restorative dentist and periodontist optimizes the prognosis for long-term success in the event of biologic complications.
The biologic complications of peri-implant disease—particularly when it progresses from peri-implant mucositis to peri-implantitis—is a significant problem that threatens the survival of dental implants and possibly their status as the standard of care for edentulism. In part, this is because of confusion in the terminology used to characterize both the disease at hand and its severity. The importance for more stringent follow-up care for dental implants versus teeth is something that is often unrecognized or understated, as there is a long-standing belief that implants are immune to complications. However, the lack of a protective mechanism, such as inserting supracrestal gingival fibers, that is present on teeth but not on dental implants makes the care for these replacements all the more critical. It is also important to recognize which patients pose the highest risks for developing these complications. But, the overriding issue that confounds early and effective treatment is the lack of clearly established guidelines for knowing which level of disease requires the need for aggressive intervention, and how to best diagnose and assess any instituted measure(s) of care.
Given what is known about the etiology of peri-implant mucositis and peri-implantitis and the importance of rendering treatment at the earliest possible stage, there are steps that clinicians should take to prevent and minimize the negative sequelae of these biologic complications. Peri-implant diseases present in two forms: peri-implant mucositis (Figure 1 and Figure 2) and peri-implantitis (Figure 3 and Figure 4). Both involve an inflammatory reaction in the tissues surrounding an implant—ie, BOP and/or suppuration and probing depths usually exceeding 4 mm.1,2 However, when there is also bone loss present beyond the physiologic remodeling that may occur after implant placement, a diagnosis of peri-implantitis should be made because this condition, when compared to mucositis, is far more serious and difficult to resolve.3
It has been reported that approximately 10% of implants will develop peri-implantitis.4 However, it is difficult to establish a definite prevalence because of significant variations in the diagnostic threshold; specifically, a designated amount of bone loss—as well as the data needed to determine when specified criteria are met are not consistently in agreement.
Distinct differences in the incidence and prevalence of peri-implantitis have been reported by a number of authors using different thresholds of radiographic bone loss as a criteria of established disease.5 Clearly, the result of this inconsistency of a diagnostic threshold used in these studies has been a broad range of incidence and prevalence of the disease reported.6-8 Because of this variation in bone loss that has been used to define the stages of peri-implantitis in published studies, the authors have proposed a simple classification of early, moderate, and severe peri-implantitis that is determined by the percentage of bone loss related to the length of the implant.9
Causes of Peri-implant Disease
What is clear is that the inflammatory process of peri-implant mucositis around an implant is somewhat similar to gingivitis around natural teeth, with one major difference in the containment of the inflammation process. While teeth have supracrestal connective tissue fibers inserting into both their root surface and the surrounding circular fibers, dental implants have only the latter. As gingivitis is often believed to precede periodontitis, mucositis is the precursor of peri-implantitis, and although both do not necessarily progress beyond a contained lesion, dental implants are less resistant to any inflammation in the connective tissue and, thus, are more susceptible to disease progression.1
The etiology of peri-implant disease has been linked to the formation of biofilm on the implant surface after its exposure to the oral environment.1,2,6-12 One recent publication proposed several co-factors, including occlusal forces, damage to the implant surface, and corrosion, that could work synergistically with bacteria to progressively increase the loss of supporting bone.13
Risk Factors
A number of risk factors associated with the establishment and progression of peri-implant mucositis and peri-implantitis were reported in the Consensus Report of the Sixth European Workshop on Periodontology.2 In addition to periodontal disease, the identified risks included poor oral hygiene; cigarette smoking; diabetes with poor metabolic control; alcohol consumption; genetic traits; and poor plaque control or the inability to clean the implant because of its placement or design.2,8
Risk factors suggested by others include occlusal overload, which, like peri-implantitis itself, has not yet been precisely defined.14 Other potential risk factors that may impact the development and pathogenesis of peri-implant bone loss may include rheumatoid arthritis with concomitant connective tissue disease15 and iatrogenic cementation, which is the incomplete removal of cement left in the subgingival space around dental implants. This problem has been described by various investigators including Wilson16, Wadhwani et al,17 and Linkevicius et al.18
Prevention/Early Intervention
In view of what is known about peri-implantitis—its commonalities and association with periodontitis, the various degrees of bone loss, and the risk it poses to implant survival—every effort should be made to prevent peri-implant mucositis. Patients should be carefully screened for risk factors. It is especially important to resolve pre-existing periodontal disease before implant placement, as suggested by studies showing that residual pockets harbor periodontal pathogens that can infect other sites.19,20 In keeping with other risk factors, practitioners should also ensure that patient plaque control and the design and placement of implants can support the level of hygiene needed to prevent the formation of biofilm.
Perhaps the most important distinction between peri-implant mucositis and peri-implantitis in terms of their clinical implications is that peri-implant mucositis has been shown to respond to nonsurgical treatment in some instances,21 while peri-implantitis does not.22 Crucial to diagnosis at the earliest stage is meticulous record-keeping and close monitoring to gather the pertinent information regarding bone and tissue abnormalities that signal the onset of peri-implant mucositis or its progression to peri-implantitis.8
Radiographs
The authors believe that in accordance with the American Academy of Periodontology’s whitepaper,8 the clinician should obtain radiographs that establish baseline bone levels both at the time of implant placement and immediately after insertion of the final prosthesis to facilitate comparison.
Probing, Bleeding, Suppuration
Initial probing of the implant should be done once the final restoration has been inserted and can be done with either a plastic or metal periodontal probe. The key to consistency is to use light force with the same type of probe. Probing depth should be recorded and defined as the depth of probe penetration from the base of the implant sulcus to the crest of the gingival tissue surrounding the implant. Similar to assessment around natural teeth, the level of the crestal soft tissue can be measured using a fixed reference point on the restoration and should be recorded as the clinical attachment level.
Recall Visits
The authors suggest that clinicians employ methods that monitor implant health and determine inflammatory complications as part of an ongoing periodontal maintenance program. This should include a comprehensive periodontal and implant examination. Because implants differ from teeth, they should be subject to different ongoing care, with more frequent recall visits than the standard once or twice per year, perhaps alternating between the restorative dentist and periodontist. Moreover, in patients with known risk factors, it becomes imperative that their visits be increased to three to four times per year.
Implant Management
Treatments aimed at controlling inflammation and infection and limiting biologic complications for peri-implant mucositis and peri-implantitis, respectively, are identical to those that have been found to be effective for gingivitis and periodontitis, respectively. Similar to gingivitis, when detected early peri-implant mucositis can be successfully treated with effective nonsurgical efforts aimed at elimination of the biofilm from the implant surface21,23,24 (Figure 5 through Figure 7), such as supportive periodontal therapy and mechanical nonsurgical treatment.
However, these methods have been reported to be far less effective once the condition has progressed to peri-implantitis. Moreover, flap-access surgical approaches with or without targeted antimicrobial treatment has been met with limited success.25,26 In the authors’ opinion, surface decontamination must completely eradicate the biofilm, debris, and excess cement. Recently, evidence has suggested that a dual antibiotic approach may be more appropriate rather than a single medication.27
The authors, both of whom are periodontists, believe that dentists should make every effort to save a failing tooth before replacing it with an implant because implants are not immune to complications and do not integrate 100% of the time. Moreover, the authors consider efforts to save implants far preferable to extraction and replacement with another implant given the time and the cost for such treatment. They, along with their colleagues, have successfully achieved improvement in bone loss for both teeth28 and implants with a regenerative approach that emphasizes surface decontamination. Dental implants, with their roughened surface topography that is designed to enhance osseointegration, require that this comprehensive regenerative approach employ these tactics: aggressive surface decontamination with mechanical and chemotherapeutic approaches; the administration of systemic antibiotics; regenerative therapy with bone and bone replacement grafting; and the use of biologics and barrier membranes, all followed by a tightly controlled maintenance approach (Figure 8 through Figure 11). In fact, in one case series article this approach to care has demonstrated promising results for up to 8.5 years postsurgery.29,30 This therapy is quite complex and significant experience is essential if success is to be consistently achieved.
For general practitioners who place implants, part of planning for success may well mean an extensive evaluation for risk factors and collaborating with a periodontist, especially for patients where the risk is high for developing peri-implant diseases. This may be especially true if the physiologic modeling and loss of marginal bone after implant placement results in the implant’s roughened surface being exposed. Patients must be informed that implants are not teeth and may require more frequent maintenance care for their long-term health. Alternating recall visits may be essential where both the dentist and the specialist take the time and care to update records and ensure that these sites have been thoroughly debrided.
Conclusion
As with many inflammatory diseases, the best treatment outcomes are associated with early diagnosis and treatment. Toward that goal, clinicians should plan for success with implants by following guidelines designed to prevent or minimize the potential for the development of peri-implant disease and, if necessary, be prepared to effectively diagnose and treat it.8 This means screening patients for risk factors associated with developing peri-implant diseases; taking care to fully treat periodontal disease, which can infect implant sites; employing methods that support and monitor implant health and recognize inflammatory complications as part of an ongoing periodontal maintenance program; and keeping careful records to track changes, including a baseline radiograph at the time of implant placement, and another at final prosthesis insertion. In many cases, treatment by a periodontal specialist, trained in regenerative therapy, may be indicated for implants with moderate to severe peri-implantitis. It is through collaboration that all clinicians can provide the best that dentistry has to offer today.
References
1. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63-76.
2. Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(Suppl 8):282-285.
3 Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11-25.
4. Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(Suppl 6):67-76.
5. Tomasi C, Derks J. Clinical research of peri-implant diseases–quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri-implant diseases. J Clin Periodontol. 2012;39(Suppl 12):207-223.
6. Roos-Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. Clin Periodontol. 2006;33:290-295.
7. Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81:231-238.
8. American Academy of Periodontology Task Force on Peri-implantitis. Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013;84:436-443.
9. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32:533-540.
10. Augthun M, Conrads G. Microbial findings of deep peri-implant bone defects. Int J Oral Maxillofac Implants. 1997;12:106-112.
11. Salcetti JM, Moriarty JD, Cooper LF, et al. The clinical, microbial, and host response characteristics of the failing implant. Int J Oral Maxillofac Implants. 1997;12:32-42.
12.Leonhardt A, Berglundh T, Ericsson I, Dahlén G. Putative periodontal pathogens on titanium implants and teeth in experimental gingivitis and periodontitis in beagle dogs. Clin Oral Implants Res. 1992;3:112-119.
13. Mouhyi J, Ehrenfest DMD, Albrektsson T. The peri-implantitis: implant surfaces, microstructure, and physiochemical aspects. Clin Implant Dent Relat Res. 2012;14:170-183.
14. Fu JH, Hsu YT, Wang HL. Identifying occlusal overload and how to deal with it to avoid marginal bone loss around implants. Eur J Oral Implantol. 2012;5(Suppl):S91-S103.
15. Krennmair G, Seemann R, Piehslinger E. Dental implants in patients with rheumatoid arthritis: clinical outcome and peri-implant findings. J Clin Periodontol. 2010;37:928-936.
16. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80:1388-1392.
17. Wadhwani C, Rapoport D, La Rosa S, et al. Radiographic detection and characteristic patterns of residual excess cement associated with cement-retained implant restorations: a clinical report. J Prosthet Dent. 2012;107:151-157.
18. Linkevicius T, Puisys A, Vindasiute E, et al. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2013;24:1179-1184.
19. Levy RM, Giannobile WV, Feres M, et al. The effect of apically repositioned flap surgery on clinical parameters and the composition of the subgingival microbiota: 12-month data. Int J Periodontics Restorative Dent. 2002;22:209-219.
20. Matuliene G, Pjetursson BE, Salvi GE, et al. Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol. 2008;35:685-695.
21. Heitz-Mayfield L, Salvi GE, Botticelli D, et al. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22:237-241.
22. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontology. 2009;36:1600-1605.
23. Pontoriero R, Tonetti MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5:254-259.
24. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182-190.
25. Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33:296-301.
26. Leonhardt A, Dahlén G, Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol. 2003;74:1415-1422.
27. Rams TE, Degener JE, van Winkelhoff AJ. Antibiotic resistance in human chronic periodontitis microbiota. J Periodontol. 2014;85:160-169.
28. Rosen PS, Toscano N, Holtzclaw D, Reynolds MA. A retrospective consecutive case series using mineralized allograft with recombinant human platelet-derived growth factor BB to treat moderate to severe osseous lesions. Int J Periodontics Rest Dent. 2011;31:335-341.
29. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3 to 7.5 year follow-up. Int J Periodontics Restorative Dent. 2012;32:11-20.
30. Froum SJ, Rosen PS. Re-entry evaluation following treatment of peri-implantitis with a regenerative approach. Int J Periodontics Restorative Dent. 2014;34:47-59.
About the Authors
Paul S. Rosen, DMD, MS
Clinical Associate Professor of Periodontics
University of Maryland Dental School
Baltimore, Maryland
Private Practice
Yardley, Pennsylvania
Stuart J. Froum, DDS
Clinical Professor and Director of Clinical Research
Department of Periodontology and Implant Dentistry
New York University College of Dentistry
New York, New York
Private Practice
New York, New York