Monolithic Versus Bilayered Restorations: A Closer Look
Abstract
The all-ceramic crown was developed in the early 20th century when Charles H. Land patented the all-porcelain “jacket” crown to improve esthetics. This procedure consisted of rebuilding the missing tooth with a porcelain covering, or “jacket” as Land called it. To solve the product’s strength problems, Abraham Weinstein in the late 1950s introduced a metal core to which porcelain was fused to the coping, thus creating the ceramo-metal crown. Throughout the years, the metal has been substituted with different materials to achieve a more esthetic result. Problems have been reported with the fusion between the ceramic and the core, which have resulted in debonding of the veneered ceramic. Further investigations in dental material science have produced tremendous advances in unveiling aspects that have been taken for granted, such as the bond strength between different materials that comprise the crown restoration. Recently, a lithium-disilicate material that was once used solely as a core material has been introduced as an all-ceramic alternative. This article discusses the strength factors that comprise a monolithic and bilayered ceramic restoration.
Advances in the field of dental ceramics can take time to find acceptance in the dental community. Ceramic materials are usually employed in higher-end procedures, and the clinician is compelled to deliver a product that has a proven durable and esthetic track record. Materials and procedures must have the science behind them and the endorsement of leading clinicians before being introduced. Only then can these materials become part of the dentist’s restorative armamentarium.
For years, the ceramo-metal restoration has been the gold standard in crown-and-bridge procedures. Although durable and time-tested, this type of restoration may not be the most esthetic. For years, patients have asked for metal-free restorations, and the industry has accommodated this request with various resin composite and ceramic systems.
These newer systems have an effect on the actual fabrication methods. Traditionally, the ceramo-metal restoration is constructed by casting a metal coping and applying a porcelain opaque layer followed by layering veneering porcelain. Newer methods have bypassed the coping fabrication step. Using a vacuum-pressing system, all-ceramic restorations are waxed to full contour and invested, wax burnt out, and hot-pressed, creating a solid ceramic restoration. The question remains if these all-ceramic monolithic forms can endure the rigors of an intraoral restoration as well as the bilayered porcelain-to-metal kind.
All-Ceramic Crowns: Bilayered versus Monolithic
Numerous bilayered crown systems that are supported by a substructure core are available. Various materials are used to create these substructures, eg, metal alloys, alumina, and zirconia. Often, ceramo-metal crowns have been used because of their strength, biocompatibility, and esthetics.1 Patient demand for more esthetic restorations has gradually increased, leading to greater use of nonmetallic, high-strength core materials. These esthetic core materials include alumina, zirconia, zirconia-toughened alumina, magnesium aluminate spinel, and lithium disilicate. Once the cores are fabricated, the laboratory technician applies veneering porcelain to create the final esthetic restoration.2,3 Yet all of these porcelain-laminated systems share a common mode of failure: fracture of the veneering ceramic from its core.
There are three basic configurations for restorative crowns: bilayered ceramo-metal, bilayered ceramo-zirconia, and monolithic lithium disilicate. While numerous studies are cited in the literature, specific comparative tests uniformly conducted on all three systems are difficult to find. The testing methods, sample sizes, and the instrumentation used in the studies are variables that must be considered. Therefore, strength comparisons of different studies can be misleading. However, after reviewing the body of literature, a different perspective may be gained as to the overall strength of each system, rather than one particular asset.
Ceramo-Metal Restorations
Through the years, replacement and reinforcement of the human tooth has evolved from a monolithic design (gold crown) to a bilayered design (ceramo-metal and ceramo-zirconia) and again to a monolithic design (lithium disilicate). In the past several decades, the workhorse restoration is the ceramo-metal crown: a metal substructure in which ceramic material is layered or pressed to form the anatomic shape of the restoration (Figure 1 and Figure 2). The weakest point is the ceramic–metal interface. The exact mechanism of porcelain-to-metal fusion is unknown; however, at least four theories have been discussed. The theory of van der Waals forces4refers to the bonding of materials created by the attraction of charged atoms that do not exchange electrons. These secondary forces are generated more by a physical attraction between charged particles than by an actual sharing or exchange of electrons in primary (chemical) bonding.5
The theory of mechanical retention of ceramic to a metal coping is derived from the microscopic irregularities. The contribution of micromechanical bonding may be relatively limited because ceramic does not require a roughened area to bond.5 Lacy4has shown that ceramic will fuse to a well-polished metal surface; however, some surface roughness does contribute to an increased bond.6-8 Therefore, mechanical retention alone is probably not sufficient to entirely explain how dental ceramic adheres to a metal substrate.5
Bonding of porcelain to metal by means of compression is the third theory. Dental porcelain, like most brittle materials, is strong in compression but relatively weak when subjected to tensile stresses. Its tensile strength is approximately 4% of its compressive strength.9 Compressive stress in the layering porcelain reinforces the fracture strength. A thermal mismatch between the coping and the porcelain leads to compressive or tensile stress depending on whether the coefficient of thermal expansion of the porcelain is higher or lower than that of the coping.10 The expansion of the porcelain must be lower than that of the coping to generate compressive stress during cooling.11 The development of compressive forces in the porcelain and tensile forces in the metal is due to the difference in contraction rates.
Chemical bonding is the final generally accepted theory as the primary mechanism of ceramic to metal attachment.12-14 The mode of bonding involves the metal surface oxides dissolved by the applied ceramic opaque layer. This results in an atomic contact whereby shared electrons form ionic and covalent bonds between the oxide layer on the metal surface and the ceramic opaque layer.12,13
Ceramo-Metal Failures
The literature cites studies observing various ceramo-metal failures. Failure rates range between 5% and 10% over 10 years.15 Strub et al found failure rates of ceramo-metal restorations as high as 3% over 5 years.16 Hankinson and Cappetta17 and Kelsey et al18 found a failure rate between 2% and 4% that occurred after 2 years. They also found that, due to a repetition of consistent occlusal contacts, after 4 to 5 years the failure rate rose to 20% to 25%.
A ceramo-metal failure is a multifactorial problem related to a combination of reasons19 (Figure 3). Some studies attribute failures to environmental factors, particularly moisture. A moist environment was found to reduce the ceramo-metal strength by 20% to 30%.20 In the presence of moisture, the silicon–oxygen bond between metal and ceramic weakens and promotes failure because of water propagation at the crack tip.21 Most frequently, ceramic failures are related to the cracks in the ceramic.19 Small scratches on the ceramic surface can act as notches where the concentration of stress can exceed the theoretical strength of the ceramic. As the crack propagates through the material, the stress concentration is maintained at the crack tip until the crack moves completely through the material.22
Technical errors in the laboratory can also account for ceramic–metal failures. A void or pore that remains after the fabrication can be the site of weakness and eventual failure.23 Porosity does occur between ceramic particles during the ceramic application, and the technician should make every effort to minimize this.
Diaz-Anold et al found several reasons for failure, including faulty metal structure design and incompatible coefficients of thermal expansion between the metal and the ceramic material.24 Another reason was insufficient metal support for the ceramic, leading to unsupported excessive thickness of ceramic, technical flaws in the porcelain application, and occlusal forces or trauma. Ceramic material properties, including microstructure, crack length, fracture toughness, and applied stress intensity, also contribute to failure.24
Usually, a catastrophic failure is the result of crack initiation and propagation. Llobell et al described reasons for intraoral ceramic failure: impact load, fatigue load, improper design, and microdefects within the material. They also found that masticatory repetitive forces, including parafunctional occlusion, created alternating forces, contributing to the fatigue of ceramo–metal restorations.25 Typically, one factor alone does not cause ceramo-metal catastrophes; rather, the cumulative effect of a large number of comparatively small loadings leads to failure.19
Bond Strength of Porcelain to Metal
The ideal test to determine the bond strength between ceramics and metal does not exist, although several methods have been used.26 Several tests have been employed to evaluate the ceramo-metal bond strength:27 shear test (maximum stress that a material can withstand before failure in shear),28 planar shear test (opposing forces are applied parallel to the cross-sectional area under test),26 tensile,29 flexural,30 and torsional strength.31 Chong and Beech28 proposed the circular-planar surface shear test, which provided standardization and ease in specimen fabrication.32
Scolaro et al27 tested different ceramics that were bonded to a palladium–silver alloy (Pors-on 4, Degussa SA, https://www.degussa.com). They used Ceramco (DENTSPLY Ceramco, https://www.ceramco.com), Noritake Super Porcelain EX-3 (Noritake Kizai, https://www.noritake-dental.co.jp), and Vita VMK® 68 (Vident, https://www.vident.com). The shear bond strength results were: Noritake (28.96 MPa ± 6.92 MPa), Ceramco (28.20 MPa ± 8.65 MPa), and Vita VMK 68 (24.11 MPa ± 6.27 MPa).
Akova et al33 compared the bond strength of layering porcelain to cast Ni-Cr and Co-Cr alloys to laser-sintered Co-Cr alloy. In this study, the mean shear bond strength was the highest for the base metal Ni-Cr (81.6 MPa ± 14.6 MPa) and slightly less for the Co-Cr base metal (72.9 MPa ± 14.3 MPa). The shear bond strength of the laser-sintered Co-Cr metal was 67 MPa ± 14.9 MPa.
Joias et al32 tested the shear bond strength of a ceramic to five commercially available Co-Cr alloys. The same ceramic (Vita Omega 900, Vident) was bonded to each alloy. The shear bond strength test was performed in a universal testing machine with a crosshead speed of 0.5 mm/min. The ultimate shear bond strength ranged from 61 MPa to 96 MPa.
According to Powers and Sagaguchi,26 an adequate bond occurs when the fracture strength or fracture stress (the stress at which a brittle material fractures) is above 25 MPa. Other studies also have accepted a sufficient bond for metal–ceramics when the fracture stress is greater than 25 MPa.19,34-36 Because this value represents the limit of the test, it could be argued whether this were a true representation of adequacy.37 As previously noted, some ceramo-metal systems in other studies have tested higher.
A recently introduced laboratory method of ceramic application to metal is the use of the lost-wax technique in which a pressable ceramic is applied to an opaque metal or zirconia core (Figure 4, Figure 5, Figure 6, and Figure 7). This is a simpler and quicker method than the conventional technique and eliminates the need for the 20% shrinkage compensation with traditional porcelain firing.38
Venkatachalam et al39 compared the debond/crack initiation strength of a leucite-based low-fusing ceramic-pressed-to-metal and feldspathic porcelain-fused-to-metal. The metal specimens included gold–palladium alloy and chrome–cobalt base metal alloy divided into two groups of 20 samples. The mechanical testing method used in this study was the Schwickerath crack-initiation three-point bending test standardized by the International Organization for Standization (ISO 9693:1999),40 which is now considered the gold standard for examining metal–ceramic bond strength.39 Their findings showed a mean debond strength for feldspathic porcelain to the base metal alloy of 36.11 MPa ± 2.31 MPa while the feldspathic porcelain to the gold–palladium alloy demonstrated a mean bond strength of 42.64 MPa ± 1.94 MPa. For the ceramic pressed-to-metal specimens, the mean debond strength of the base metal combination was 37.47 MPa ± 6.02 MPa and 47.94 MPa ± 3.92 MPa for the gold–palladium samples.
Ceramo-Zirconia Failures
The actual mechanism of bonding ceramic to zirconia substructures is not completely understood, nor is the manipulation of surface treatment of zirconia in the quality of the bond.41-43 Sufficient bond strength between veneering ceramic and zirconia framework substructures is a concern for long-term success.44 Chipping of the veneering ceramic constitutes clinical failure and has been reported to occur at a rate of 13% during a 3-year observation.45 In a follow-up study, Sailer et al found the failure rate increased to 15.2% during a 5-year period.46
One approach to enhancing ceramic-to-zirconia bond strength is sandblasting, which increases the surface roughness and provides undercuts.39-41 Conversely, Kosmac et al47 and Guazzato et al48 found sandblasting adversely affects the mechanical strength of the zirconia by initiating a phase transition (tetragonal to monoclinic form) and probably has a detrimental effect on the bonding capacity. This phase transition of tetragonal zirconia to monoclinic zirconia results in a significantly lower coefficient of thermal expansion.
Fischer et al49 investigated the effect of different surface treatments on the bond strength of veneering ceramics to zirconia. Their study assessed the influence of treating the zirconia surface by polishing, sandblasting, silica-coating, and applying a liner. They also studied the impact of regeneration firing, which entails firing the zirconia framework for 15 mins at 1,000° C prior to veneering. This re-establishes the tetragonal lattice after sandblasting or grinding to obtain better bond strength.50 Five different layering ceramics were used. The shear strength of all the types of surface conditions was 23.5 MPa ± 3.4 MPa to 31 MPa ± 7.1 MPa. In all specimens, the fracture started at the core–veneer interface and continued into the veneering ceramic, which remained on the core. The weakest link was not the interface, but the veneering ceramic itself. This study concluded that increased surface roughness did not enhance shear strength, the application of a liner did not improve shear strength, and regeneration firing decreased the shear strength. The recommendation to realize the benefit of high-strength zirconia as a framework was to strengthen the veneering ceramic.
Although the zirconia substructure is fracture-resistant, a high percentage of failures of the ceramo-zirconia restoration are found in ceramic chipping and delamination.51-53
A randomized, controlled clinical trial showed the performance of three-unit posterior prostheses using three ceramo-metal fabrication methods and five major companies’ zirconia technologies.54 The researchers evaluated both the framework and the veneering ceramics. The report showed veneering ceramic fractures were five times more prevalent with ceramic formulations used on zirconia versus those employed on metal.
In another study, Taskonak et al55 determined the site of crack initiation and the causes of fracture in failed zirconia-based ceramic fixed partial dentures. Fractures that had origins on the ceramic veneer surface had failure stresses between 31 MPa and 38 MPa.
Aboushelib et al56 stated that the bond strength between veneer ceramic and the zirconia framework is the weakest component in the layered structure. To enhance the final esthetics of layered zirconia-based restorations, colored pigments are incorporated into the surface of the zirconia framework (Figure 8). The objective of this study was to investigate the effect of zirconia type (white or colored) and its surface finish on the bond strength to two veneer ceramics. They found the addition of coloring pigments resulted in a significantly weaker bond strength compared to the white zirconia frameworks.
In their comparative study, Guess et al57 evaluated the shear bond strength between various commercial zirconia core and veneering ceramics and the effect of thermocycling. Using the Schmitz-Schulmeyer test method, they evaluated the core–veneer shear bond strength of Cercon® Base to Cercon Ceram S (DENTSPLY, https://www.dentsply.com); Vita In-Ceram® YZ cubes to Vita VM9 (Vident); and DC-Zirkon to IPS e.max® Ceram (Ivoclar Vivadent, https://www.ivoclarvivadent.us). As a control specimen, they used a ceramo-metal system, Degudent U94 (Degudent, https://www.degudent.com) to Vita VM13. Half of each specimen group was thermocycled at 5°C to 55°C for 20,000 cycles. Their results demonstrated the shear bond strength values of 12.5 MPa ± 3.2 MPa for Vita In-Ceram YZ Cubes/Vita VM9, 11.5 MPa ± 3.4 MPa for DC-Zirkon/IPS e.max Ceram, and 9.4 MPa ± 3.2 MPa for Cercon Base/Cercon Ceram S. The specimens that were thermocycled did not show any significant differences. The control ceramo-metal specimen showed a higher shear bond strength, regardless of thermocycling, of 27.6 MPa ± 12.1 MPa.
Monolithic Restorations: Lithium Disilicate
The first all-ceramic restorative system was introduced in 1903 by Charles Land.58 The so-called “porcelain jacket crown” was fabricated with high-fusing feldspathic porcelain. Although it was noted for natural esthetics, the failure rate was high, probably due to the low strength of the porcelain.59 Interest in all-ceramic restorations has grown throughout the years. These developments have included several bilayered systems consisting of a ceramic-type substructure interfaced with a veneering ceramic.
Recently, a monolithic approach was introduced using lithium-disilicate glass ceramic (eg, IPS e.max Press and IPS e.max CAD). This material has two forms: a homogeneous ingot with various degrees of opacity used with hot-pressed technology and a precrystallized block used with CAD/CAM technology. Both forms can be used in a full anatomical contour method with the application of stain and glaze or a cut-back and layering technique.
The milling blocks for the CAD version are produced for distribution using a glass technology. This process prevents the formation of defects and voids throughout the block and allows for an even distribution of the pigmentation. This partial crystallization process forms lithium-metasilicate crystals, which provide sufficient strength for milling. According to the manufacturer, the partially crystallized milling block has a microstructure consisting of 40% lithium-metasilicate crystals, which are embedded in a glassy matrix. The grain size of these crystals ranges from 0.2 µm to 1 µm. At this point, the lithium-metasilicate block has a flexural strength of 130 MPa, which is comparable to leucite-reinforced CAD/CAM (ProCAD, Ivoclar Vivadent) blocks and feldspathic CAD/CAM blocks (Vitablocs® Mark II, Vident).60 After milling, the precrystallized restoration is placed in the mouth and adjusted, if necessary. The restoration is then crystallized during a 20-minute firing cycle using a two-step ceramic furnace. As the restorations can be milled to full contour, there is no ceramic infiltration process or veneering process. Although there is 0.2% shrinkage during the crystallization process, the computer software accounts for this in the milling process. During the crystallization cycle, the lithium-metasilicate restoration reaches a temperature of 840°C to 850°C (1,544°F to 1,562°F). During the temperature rise, a controlled growth of lithium-disilicate crystals occurs, producing a transformation of the microstructure that results in an increase of the final flexural strength of 360 MPa. This flexural strength is approximately three to four times stronger than leucite-reinforced glass ceramics.61
This glass ceramic is comprised of 70% prismatic lithium-disilicate crystals (0.5 µm to 5 µm long) dispersed in a glassy matrix.62 The lithium-disilicate microstructure has numerous small interlocking plate-like crystals randomly oriented. This crystal size and orientation causes cracks to deflect, branch, or blunt, which can account for the increase in flexural strength and fracture toughness compared to leucite-reinforced ceramics.63
The manufacturer’s internal testing (Ivoclar Vivadent, unpublished data, 2005) states the fracture toughness (single-edge, V-notched beam testing) to be 2 MPa to 2.5 MPam1⁄2 and a modulus of elasticity of 95 GPa ± 5 GPa. Bindl et al64 studied the fracture strength and fracture pattern of three monolithic posterior crowns (lithium disilicate, leucite glass, and feldspathic ceramic) that have a uniform thickness of 1.5 mm. They conventionally cemented one half of the specimens while adhesively cementing the other half on dies. For the conventionally cemented crowns, load to fracture was 2,082 N, which was significantly higher than that of the leucite glass or feldspathic ceramic. When the specimens were adhesively cemented to the die, the fracture load for the lithium disilicate rose to 2,389 N, which was comparable to the two other specimens. This study showed the strength of the lithium disilicate when conventional cementing techniques are employed. A manufacturer’s internal study comparing the difference in failure load for monolithic and bilayered crowns showed adhesively retained monolithic lithium-disilicate restorations had the highest load to failure numbers.69,70
The pressed form of the lithium disilicate has been shown to have a modulus of elasticity ranging from 91 GPa67 to 95 GPa ± 5 GPa (Ivoclar Vivadent AG, unpublished data 2009). The flexural strength varies depending on the testing method used. Using biaxial flexural strength tests under dry and wet conditions, Sorenson et al68 found a flexural strength ranging from 411.6 MPa to 455.5 MPa. Albakry et al67 measured the biaxial strength with a universal testing machine. Twenty standardized disc specimens (14 mm x 1.1 mm) were supported on three balls and loaded with a piston at a crosshead speed of 0.5 mm/min until fracture. The mean biaxial strength for the lithium-disilicate specimen was 440 MPa ± 55 MPa.
Depending on the testing method, fracture toughness of the lithium disilicate has been shown to be at least or greater than 3 MPa m1⁄2. Using the indentation strength technique, Guazzato et al69 found a fracture toughness of 3 MPa m1⁄2. Albakry et al70 measured the fracture toughness of the pressed lithium disilicate using two different techniques: indentation fracture and indentation strength. They reported a fracture toughness of 3.14 MPa and 2.5 MPa m1⁄2, respectively.
Veneering Ceramic for Lithium Disilicate
The coefficient of thermal expansion of feldspathic glass is closely matched to alumina-based core material (~7 ppm/°C to 8 ppm/°C) and, consequently, can be used as a veneering ceramic. Leucite layering ceramics have the same coefficient of thermal expansion as the leucite core material, therefore, posing no problems in coefficient mismatch. However, the coefficient of thermal expansion of lithium disilicate is greater than 10 ppm/°C. As a result, a new compatible layering ceramic was developed.62 The layering material (IPS e.max Ceram) is a low-fusing nano-fluorapatite glass-ceramic. It can be used with either the pressed or CAD/CAM version of the lithium-disilicate core and does not contain feldspar or leucite.
The light refraction gives the lithium-disilicate material a natural appearance and can be used in a monolithic form. In this state, the flexural strength remains throughout the entire restoration. Surface colorants are available to obtain the final shade and characterization.
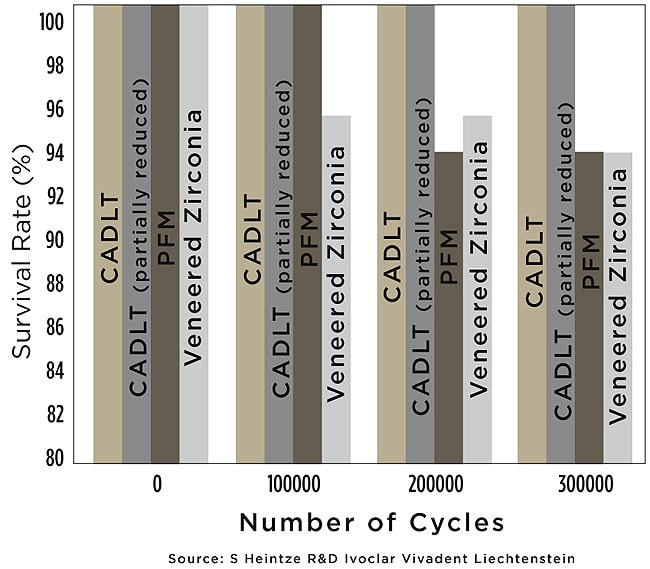
When in-depth characterization is desired, a partial-layering technique can also be employed. At this point, it can no longer be considered monolithic because it still comprises a majority of the structure compared to the zirconia-based restoration in which the bulk of the restoration is the veneered ceramic. A manufacturer’s internal long-term cyclic loading study compared various restorative dental materials for crowns with monolithic lithium-disilicate crowns with and without layered veneering porcelain.71 After 300,000 cycles, only the monolithic lithium-disilicate restorations—regardless of whether layering veneer porcelain had been applied—did not show any breakdown (Figure 9).
Conclusion
Restorative dentistry is the science and art of replacing human tooth structure. The tooth is comprised of enamel and dentin, which individually are low-strength materials but, when combined, their bond is unique and can survive a lifetime. Technology has not been able to replicate nature’s bioengineering. For many decades, the ceramo-metal crown has been the mainstay of restorative dentistry. Recently, the zirconia-based ceramic restoration was introduced with better esthetics and core strength. Both systems are bilayered restorations with the bulk of the restoration consisting of a veneered feldspathic ceramic or a leucite-reinforced, low-fusing pressed ceramic. In either case, the strength is dependent on the bond strength at the interface between the core and its ceramic veneer. A new approach has been described in which a ceramic with excellent optical properties and high flexural strength can be used in a monolithic design. The resulting restoration possesses these qualities throughout its entirety as opposed to a restoration based on a bond between two dissimilar materials—the layering ceramic and the core—in which bond strength is less than the individual parts of the crown. Because nature’s bilayered tooth structure cannot be replicated, a monolithic approach may be the future.
Acknowledgments
The author would like to thank Ruth Egl, RDH, for her editorial contribution and to acknowledge Kramer Helvey for his support.
About the Author
Gregg Helvey, DDS, MAGD
Private Practice
Middleburg, Virginia
References
1. Özcan M. Fracture reasons in ceramic-fused-to-metal restorations. J Oral Rehab. 2003;30(3):265-269.
2. Spear F, Holloway J. Which all-ceramic system is optimal for anterior esthetics? J Am Dent Assoc. 2008;139(suppl 4):19S-24S.
3. Imbeni V, Kruzic JJ, Marshall GW, et al. The dentin-enamel junction in preventing the fracture of human teeth. Nat Mater. 2005;4(3):229-232.
4. Lacy AM. The chemical nature of dental porcelain. Dent Clin North Am. 1977;21(4):661-667.
5. Naylor PW. Introduction to Metal-Ceramic Technology. Chicago, Ill: Quintessence Publishing Co; 1992:83.
6. Hin TS. Engineering Materials for Biomedical Applications. Hackensack, NJ: World Scientific; 2004:5-13.
7. Mitchell L, Brunton D. Oxford Handbook of Clinical Dentistry. New York, NY: Oxford University Press; 2005:694.
8. Fairhurst CW, Rodway JM Jr, Twiggs SW, et al. In: Smothers W, ed. Proceedings of Conference on Recent Developments in Dental Ceramics: Ceramic Engineering and Science Proceedings. 2008;6(1/2):66-83.
9. Ferracane JL. Materials in Dentistry: Principles and Applications. 2nd edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2001:161.
10. Fischer J, Stawarczyk B, Tomic M, et al. Effect of thermal misfit between veneering ceramics and zirconia frameworks on in vitro fracture load of single crowns. J Dent Mater. 2007;26(6):766-722.
11. Bagby M, Marshall SJ, Marshall GW Jr. Metal ceramic compatibility: a review of the literature. J Prosthet Dent. 1990;63(1):21-25.
12. McLean JW. The Science and Art of Dental Ceramics. Volume II: Bridge Design and Laboratory Procedures in Dental Ceramics. Chicago, Ill: Quintessence; 1980.
13. Yamamoto M. Metal-Ceramics. Principles and Methods of Makoto Yamamoto. Chicago: Quintessence; 1985.
14. Murakami I, Schulman A. Aspects of metal-ceramic bonding. Dent Clin North Am. 1987;31(3):333-346.
15. Coornaert J, Adriaens P, de Boever J. Long-term clinical study of porcelain-fused-to-gold restorations. J Prosthet Dent. 1984;51(3):338-342.
16. Strub JR, Stiffler S, Schärer P. Causes of failure following oral rehabilitation: biological versus technical factors. Quintessence Int. 1988;19(3):215-222.
17. Hankinson JA, Cappetta EG. Five years’ clinical experience with leucite-reinforced porcelain crown system. Int J Periodontics Restorative Dent. 1994;14(2):138-153.
18. Kelsey WP 3rd, Cavel T, Blankenau RJ, et al. 4-year clinical study of castable ceramic crowns. Am J Dent. 1995;8(5):259-262.
19. Özcan M. Fracture reasons in ceramic-fused-to-metal restorations. J Oral Rehabil. 2003;30(3):256-269.
20. Sherrill CA, O’Brien WJ. Transverse strength of aluminous and feldspathic porcelain. J Dent Res. 1974;53(3):683-690.
21. Dauskardt RH, Marshall DB, Ritchie RO. Cyclic fatigue-crack propagation in magnesia-partially-stabilized zirconia ceramics. J Am Ceram Soc. 1990;73(4):893-903.
22. Lamon J, Evans AG. Statistical analysis of bending strengths for brittle solids: a multiaxial fracture problem. J Am Ceram Soc. 1983; 66(3):177-182.
23. Oram DA, Davies EH, Cruickshank-Boyd DW. Fracture of ceramic and metalloceramic cylinders. J Prosthet Dent. 1984;52(2):221-230.
24. Evans D, Barghi N, Malloy CM, et al. The influence of condensation method on porosity and shade of body porcelain. J Prosthet Dent. 1990;63(4):380-389.
25. Llobell A, Nicholls JI, Kois JC, et al. Fatigue life of porcelain repair systems. Int J Prosthodont. 1992;5(3):205-213.
26. Powers JM, Sakaguchi RL. Craig’s Restorative Dental Materials. 12th ed. St. Louis, Mo: Mosby; 2006:469.
27. Scolaro JM, Pereira JR, do Valle AL, et al. Comparative study of ceramic-to-metal bonding. Braz Dent J. 2007;18(3):240-243.
28. Chong MP, Beech D. A simple shear test to evaluate the bond strength of ceramic fused to metal. Aust Dent J. 1980;25(6):357-361.
29. Sced IR, McLean JW. The strength of metal-ceramic bonds with base metals containing chromium. A preliminary report. Br Dent J.1972;132(6):232-234.
30. Mackert JR Jr, Parry EE, Hashinger DT, et al. Measurement of oxide adherence to PFM alloys. J Dent Res. 1984;63(11):1335-1340.
31. Herø H, Syverud M. Carbon impurities and properties of some palladium alloys for ceramic veneering. Dent Mater. 1985;1(3):106-110.
32. Joias RM, Tango RN, Junho de Araujo JE, et al. Shear bond strength of a ceramic to Co-Cr alloys. J Prosthet Dent. 2008;99(1):54-59.
33. Akova T, Ucar Y, Tukay A, et al. Comparison of the bond strength of laser-sintered and cast base metal dental alloys to porcelain. Dent Mater. 2008;24(10):1400-1404.
34. Haselton DR, Diaz-Anold AM, Dunne JT Jr. Shear bond strengths of 2 intraoral porcelain repair systems to porcelain or metal substrates. J Prosthet Dent. 2001;85(5):526-531.
35. Coornaert J, Adriaens P, De Boever J. Long-term clinical study of porcelain-fused-gold restorations. J Prosthet Dent. 1984;51(3):338-342.
36. Özcan M, Niedermeier W. Clinical study on the reasons and location of the failures of metal-ceramic restorations and survival of repairs, Int J Prosthodont. 2002;15(3):299-302.
37. Dündar M, Özcan M, Gökçe B, et al. Comparison of two bond strength testing methodologies for bilayered all-ceramics. Dent Mater. 2007;23(5):630-636.
38. Grossman DG. Cast glass ceramics. Dent Clin North Am. 1985; 29(4):725-739.
39. Venkatachalam B, Goldstein GR, Pines MS, et al. Ceramic pressed to metal versus feldspathic porcelain fused to metal: a comparative study of bond strength. Int J Prosthodont. 2009;22(1):94-100.
40. Metal-Ceramic Bond Characterization (Schwickerath Crack Initiation Test), ISO 9693. Geneva, Switzerland: International Organization for Standardization; 1999.
41. Luthardt RG, Sandkuhl O, Reitz B. Zirconia-TZP and alumina–advanced technologies for the manufacturing of single crowns. Eur J Prosthodont Rest Dent. 1999;7(4):113-119.
42. Aboushelib MN, de Jager N, Kleverlaan CJ, et al. Microtensile bond strength of different components of core veneered all-ceramic restorations. Dent Mater. 2005;21(10):984-991.
43. Aboushelib MN, Kleverlaan CJ, Feilzer AJ, et al. Microtensile bond strength of different components of core veneered all-ceramic restorations. Part II: zirconia veneering ceramics. Dent Mater. 2006;22(9):857-863.
44. Fischer J, Stawarczyk B, Tomic M, et al. Effect of thermal misfit between veneering ceramics and zirconia frameworks on in vitro fracture load of single crowns. Dent Mater J. 2007;26(6):766-772.
45. Sailer I, Fehér A, Filser F, et al. Prospective clinical study of zirconia posterior fixed partial dentures: 3-year follow-up. Quintessence Int. 2006;37(9):41-49.
46. Sailer I, Fehér A, Filser F, et al. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007;20(4):383-388.
47. Kosmac T, Oblak C, Jevnikar P, et al. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic. Dent Mater. 1999;15(6):426-433.
48. Guazzato M, Quach L, Albakry M, et al. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. Dent Mater. 2005;33(1):9-18.
49. Fischer J, Grohmann P, Stawarczyk B. Effect of zirconia surface treatments on the shear strength of zirconia/veneering ceramic composites. Dent Mater. 2008;27(3):448-454.
50. Vita Zahnfabrik. Veneering material Vita VM9 [instructions]. Bad Säckingen, Germany: Vita Zahnfabrik; 2007.
51. Vult von Steyern P, Carlson P, et al. All-ceramic fixed partial dentures designed according to the DC-Zirkon technique. A 2-year study. J Oral Rehabil. 2005;32(3):180-187.
52. Raigrodski AJ, Chiche GJ, Potiket N, et al. The efficacy of three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: a prospective clinical pilot study. J Prosthet Dent. 2006;96(4):237-244.
53. Sailer I, Pjetursson BE, Zwahlen M, et al. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part II: fixed dental prostheses. Clin Oral Implants Res. 2007;18(suppl 3):86-96.
54. PFM vs zirconia restorations—how are they comparing clinically? Gordon J. Christensen Clinicians Report. 2008;1(11):1-2.
55. Taskonak B, Yan J, Mecholsky JJ Jr, et al. Fractographic analyses of zirconia-based fixed partial dentures. Dent Mater. 2008;24(8):1077-1082.
56. Aboushelib MN, Kevelaan CJ, Feilzer AJ, et al. Effect of zirconia type on its bond strength with different veneer ceramics. J Prosthodont. 2008;17(5):401-408.
57. Guess PC, Kulis A, Witkowski S, et al. Shear bond strengths between different zirconia cores and veneering ceramics and their susceptibility to thermocycling. Dent Mater. 2008;24(11):1556-1567.
58. Land CH. Porcelain dental art. Dent Cosmos. 1903;45:437-444.
59. O’Brien WJ. Dental Materials: Properties and Selection. Chicago, Ill: Quintessence; 1989:408.
60. Giordano R. Materials for chairside CAD/CAM-produced restorations. J Am Dent Assoc. 2006;137(suppl 1):14S-21S.
61. Seghi RR, Sorensen JA. Relative flexural strength of six new ceramic materials. Int J Prosthodont. 1995;8(3):239-246.
62. Powers JM, Sakaguchi RL. Craig’s Restorative Dental Materials. 12th ed. St. Louis, Mo: Mosby; 2006:469.
63. van Noort R. Introduction to Dental Materials. Philadelphia, Pa: Elsevier; 2002:244.
64. Bindl A, Lüthy H, Mörmann WH. Strength and fracture pattern of monolithic CAD/CAM-generated posterior crowns. Dent Mater. 2006;22(1):29-36.
65. Hill TJ, et al. Cementation Effect on the Fracture Load of Two CAD/CAM Materials. 2009; Miami, FL: IADR. Abstract #0052.
66. Dasgupta T, et al. Fracture Load of Two PFM Veneering Techniques. 2008; Toronto, Canada: IADR. Abstract #2323.
67. Albakry M, Guazzato M, Swain MV. Biaxial flexural strength, elastic moduli, and x-ray diffraction characterization of three pressable all-ceramic materials. J Prosthet Dent. 2003;89(4):374-380.
68. Sorenson JA, Berge HX, Edelhoff D. Effect of storage media and fatigue loading on ceramic strength. J Dent Res. 2001;79:217.
69. Guazzato M, Ringer SP, Albakry M, et al. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part I. Pressable and alumina glass-infiltrated ceramics. Dent Mater. 2004;20(5):441-448.
70. Albakry M, Guazzato M, Swain MV. Fracture toughness and hardness evaluation of three pressable all-ceramic dental materials. J Dent. 2003;31(3):181-188.
71. Guess PC, Zavanelli R, Silva NR, Thompson VP. Clinically relevant testing of dental porcelains for fatigue and durability with an innovative mouth motion simulator. Presented at: 39th Annual Session of the American Academy of Fixed Prosthodontics. February 2009; Chicago, IL.